Jan 23, 2023We can also build sp 3 d and sp 3 d 2 hybrid orbitals if we go beyond s and p subshells. sp 2 hybridization. sp 2 hybridization can explain the trigonal planar structure of molecules. In it, the 2s orbitals and two of the 2p orbitals hybridize to form three sp orbitals, each consisting of 67% p and 33% s character. The frontal lobes align
Ultimate Quiz On Hybridisation – Trivia & Questions
Study with Quizlet and memorize flashcards containing terms like The skeletal structure of tetracyanoethylene is shown below. Determine the number of sigma (σ) and pi (π) bonds in the molecule., What is the shape of the 2p orbitals?, Which set of hybrid orbitals is represented by the picture below? picture and more.

Source Image: chemistry.stackexchange.com
Download Image
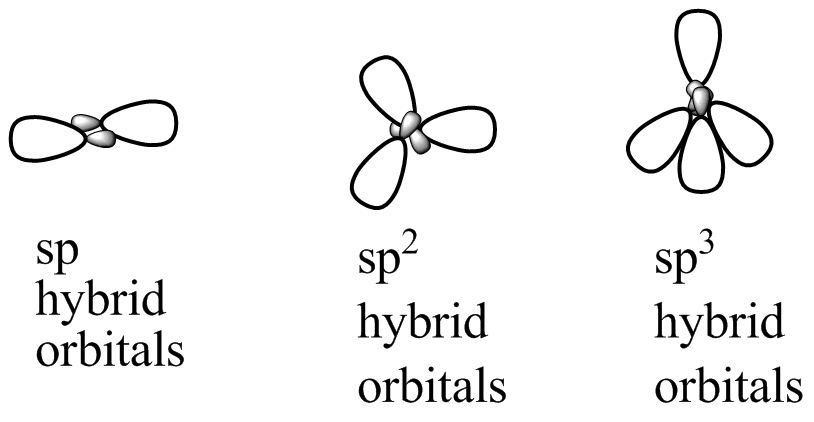
The valence orbitals of an atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs consist of a set of four sp3 hybrid orbitals. The hybrids result from the mixing of one s orbital and all three p orbitals that produces four identical sp3 hybrid orbitals (Figure 4.3.14 4.3. 14 ).
Source Image: chegg.com
Download Image
What are those smaller lobes in hybrid orbitals? – Quora Three atomic orbitals on each carbon – the 2 s, 2 px and 2 py orbitals – combine to form three sp2 hybrids, leaving the 2 pz orbital unhybridized. The three sp2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120° between them.

Source Image: chembam.com
Download Image
There Are Hybrid Orbitals Represented By This Picture.
Three atomic orbitals on each carbon – the 2 s, 2 px and 2 py orbitals – combine to form three sp2 hybrids, leaving the 2 pz orbital unhybridized. The three sp2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120° between them. Aug 22, 2022sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry (Figure \(\PageIndex5\)).
Orbitals |
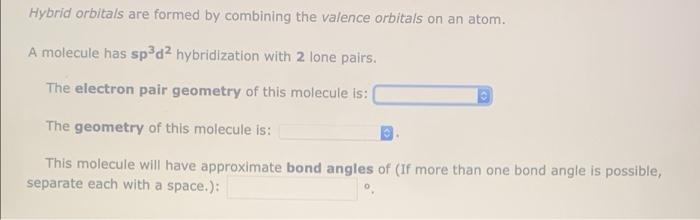
Hybrid Orbitals Hybrid orbitals are formed by combining the valence orbitals on an atom, There are hybrid orbitals represented by this picture. They are composed of atomic orbitals, corresponding to (number) hybridization. They have an electron pair geometry of With bond angles of (If more than one bond angle is possible, separate cach with a 13,385 Orbital S Images, Stock Photos, 3D objects, & Vectors | Shutterstock

Source Image: shutterstock.com
Download Image
Hybrid Orbitals – sp³ Study Guide – Inspirit Learning Inc Hybrid Orbitals Hybrid orbitals are formed by combining the valence orbitals on an atom, There are hybrid orbitals represented by this picture. They are composed of atomic orbitals, corresponding to (number) hybridization. They have an electron pair geometry of With bond angles of (If more than one bond angle is possible, separate cach with a
Source Image: inspiritvr.com
Download Image
Ultimate Quiz On Hybridisation – Trivia & Questions Jan 23, 2023We can also build sp 3 d and sp 3 d 2 hybrid orbitals if we go beyond s and p subshells. sp 2 hybridization. sp 2 hybridization can explain the trigonal planar structure of molecules. In it, the 2s orbitals and two of the 2p orbitals hybridize to form three sp orbitals, each consisting of 67% p and 33% s character. The frontal lobes align

Source Image: proprofs.com
Download Image
What are those smaller lobes in hybrid orbitals? – Quora The valence orbitals of an atom surrounded by a tetrahedral arrangement of bonding pairs and lone pairs consist of a set of four sp3 hybrid orbitals. The hybrids result from the mixing of one s orbital and all three p orbitals that produces four identical sp3 hybrid orbitals (Figure 4.3.14 4.3. 14 ).
Source Image: quora.com
Download Image
Geometrical Arrangements Characteristic of Hybrid Orbital Sets | Chemistry basics, Teaching chemistry, Molecular geometry Figure 3. Hybridization of an s orbital (blue) and a p orbital (red) of the same atom produces two sp hybrid orbitals (purple). Each hybrid orbital is oriented primarily in just one direction. Note that each sp orbital contains one lobe that is significantly larger than the other. The set of two sp orbitals are oriented at 180°, which is consistent with the geometry for two domains.

Source Image: pinterest.com
Download Image
Hybrid Orbitals and Hybridization | Organic chemistry, Organic chemistry study, Organic chemistry books Three atomic orbitals on each carbon – the 2 s, 2 px and 2 py orbitals – combine to form three sp2 hybrids, leaving the 2 pz orbital unhybridized. The three sp2 hybrids are arranged with trigonal planar geometry, pointing to the three corners of an equilateral triangle, with angles of 120° between them.

Source Image: pinterest.com
Download Image
SOLVED: There are hybrid orbitals represented by this picture They are composed of atomic orbitals, corresponding to (number) hybridization. They have an electron pair geometry of With bond angles of (If more Aug 22, 2022sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry (Figure \(\PageIndex5\)).

Source Image: numerade.com
Download Image
Hybrid Orbitals – sp³ Study Guide – Inspirit Learning Inc
SOLVED: There are hybrid orbitals represented by this picture They are composed of atomic orbitals, corresponding to (number) hybridization. They have an electron pair geometry of With bond angles of (If more Study with Quizlet and memorize flashcards containing terms like The skeletal structure of tetracyanoethylene is shown below. Determine the number of sigma (σ) and pi (π) bonds in the molecule., What is the shape of the 2p orbitals?, Which set of hybrid orbitals is represented by the picture below? picture and more.
What are those smaller lobes in hybrid orbitals? – Quora Hybrid Orbitals and Hybridization | Organic chemistry, Organic chemistry study, Organic chemistry books Figure 3. Hybridization of an s orbital (blue) and a p orbital (red) of the same atom produces two sp hybrid orbitals (purple). Each hybrid orbital is oriented primarily in just one direction. Note that each sp orbital contains one lobe that is significantly larger than the other. The set of two sp orbitals are oriented at 180°, which is consistent with the geometry for two domains.